Customer and Site Care
Partner Testimonials
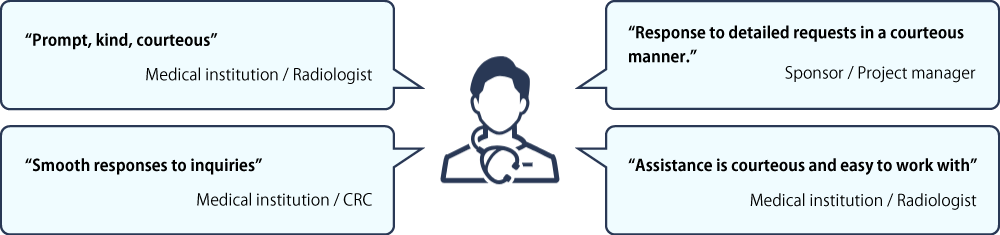
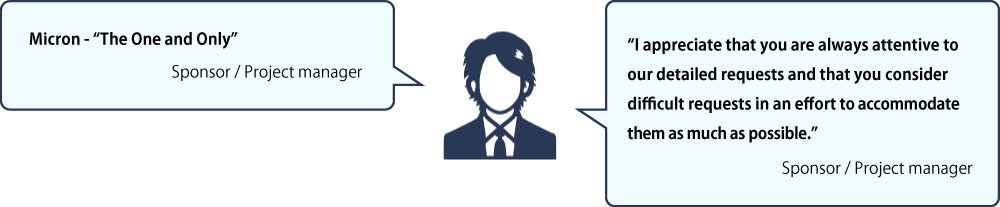
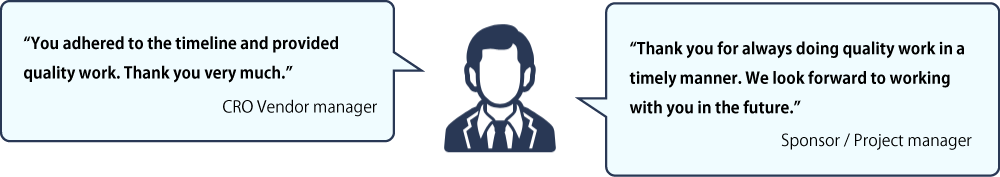
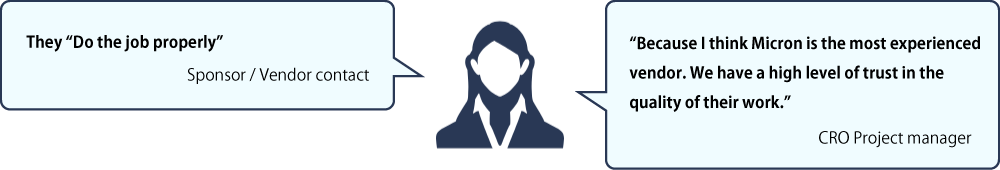
We care about and value our partners’ insights. We conducted interviews and surveys in 2020 and 2023 with our partners: key opinion leaders (KOLs), pharmaceutical companies, CROs and clinical trial sites. See below how they describe Micron. They recommend us to their colleagues in the industry.
Furthermore, we use audits and surveys to improve our company in line with our customers’ expectations and wishes.
Friendly and Courteous
Specialized
Prompt Adherence to Timelines
Accurate and High quality
Key Opinion Leader, Ophthalmologist, 2022
In ophthalmology standardized imaging formats, such as DICOM, do not exist. Generally, each ophthalmology group owns their own viewer and software with their own characteristic features. Therefore, operational know-how, with a wide variety of devices, is required for centralized ophthalmic image interpretation.
Micron owns viewer software for each manufacturer and has well trained staff to operate each viewer. Micron staff support, during centralized image interpretation, provided a stress-free environment for centralized ophthalmology image interpretation. In collaboration with Micron, the image analysis procedures minimized variability as measured by calculating inter-rater differences.
Imaging Study Rescue
Sometimes a study sponsor finds unresolvable difficulties in imaging study operations handled with their current iCRO/ICL vendor. We have often been contacted to rescue imaging studies.
Case Study
Study sponsor: Pharmaceutical Company
Study: Oncology Phase 3 Asian multi-national clinical trial
Results: Micron cleared all difficulties and concluded the project with reliable imaging study data, on-time.
Micron Rescue
Given the study problems, and the sponsor’s frustration, one of the KOLs recommended Micron to the sponsor. Although the clinical study had already started, the study sponsor decided to replace the hired iCRO with Micron. We then revamped the imaging study documentation, including the Image Review Charter, the Imaging Site Manual and Data Transfer Plan. The data transfer and reading systems, as well site support and communication methods, were changed to meet multiple local and cultural needs for each national: South Korea, Taiwan, Singapore and Japan. In addition, we customized our central review reading system to shorten read times. Ultimately Micron built strong bridging relationships with the sponsor, the Readers and the imaging sites.
Stakeholder Benefits
With Micron’s services, the imaging sites submitted compliant image data to Micron in a timely manner. This reduced the workload for the imaging sites, the CRAs and the sponsor. In addition, our new central review reading system facilitated greater reader efficiency and improved performance . Micron provided reliable data to the study sponsor, without delays, for a successful data-base lock.
Why Micron
Our focus is on customer relationships, steady and reliable internal expertise, deeply rooted in science and medicine. Micron’s rescue study experience has led us to repeat business with happy customers. Micron would be happy to manage your clinical trial’s imaging so that a rescue study is never needed.
Related Articles
Please also visit our Proactive Site Management introduction
