Our Imaging Services
Clinical trials continue to grow in complexity. Micron’s mature imaging processes can take the worry out of your trial’s medical imaging requirements. Micron manages clinical trials globally with the very specific cultural and language ingenuity in the Asia Pacific region that you need.

19+
Years of Experience

250+
Customers (total)

40+
Drugs to Market

35000+
Patients
21
Imaging Modalities

350+
Projects / Trials

200+
Medical Reader Network
From customer surveys, we know sponsors deeply value our central reading services, flexible support to imaging sites, and our experienced reader pool.
We understand and anticipate customer concerns and address them in our processes including: the need for imaging expertise, responsiveness, understanding complex imaging requirements, accuracy of results, and meeting timelines – every time, meaning fewer change requests.
We are passionate about sharing our friendly, courteous, consultative imaging services for your clinical trial. Micron is a world renowned, quality-first, thoroughly responsive iCRO (Imaging Contract Research Organization) for your global clinical trials.
Workflow
Micron provides imaging services for clinical trials based on the procedures and the FDA guidance below.
We offer best-in-class quality control and operations through all the stages of clinical trial imaging.
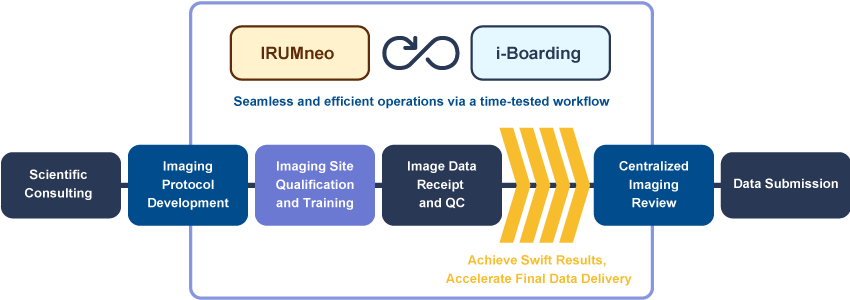
Our scientific consulting services ensure precision and trust in your clinical trials.
At Micron, our imaging protocol development is where expertise meets excellence. We collaborate with you to craft ideal imaging protocols for clinical trial success.
Navigate clinical trials with clarity. Our imaging site qualification and training guarantee the highest standards of excellence.
Our image data receipt and QC processes guarantee impeccable data quality and integrity. Experience a redefined approach to imaging.
Our centralized image review optimizes data analysis for your trials. Elevate your imaging research with our guidance and proficiency in central reads for a variety of disease areas and read criteria.
We offer ideal processes for data submission thus creating efficient clinical trials. Trust Micron to ensure your trials are a step ahead.
Micron utilizes the FDA Guidance: “Clinical Trial Imaging Endpoint Process Standards and Guidance for Industry”, which recommends standardized imaging processes for each clinical trial. In addition to daily imaging procedures, supplemental risk procedures need to be implemented. Our Proactive Site Management is just one example of how we manage common risks in imaging for clinical trials.
Contact us for further information on our workflows.
Related Articles
Our Systems
Global Reach
IRUM and i-Boarding are registered trade marks of Micron, Inc.
