Our Quality
Risk Based Quality Management
Risks in clinical trials should be considered at the systems level (e.g. facilities, standard operating procedures, computerized systems, personnel, vendors), as well as at the trial level (e.g. trial design, data collection and recording).
Our qualified experts have managed the risks in imaging and image-analysis for multi-site global clinical trials for decades. Our highly trained, multilingual, experienced, technical staff have a low turnover rate to better serve your studies.
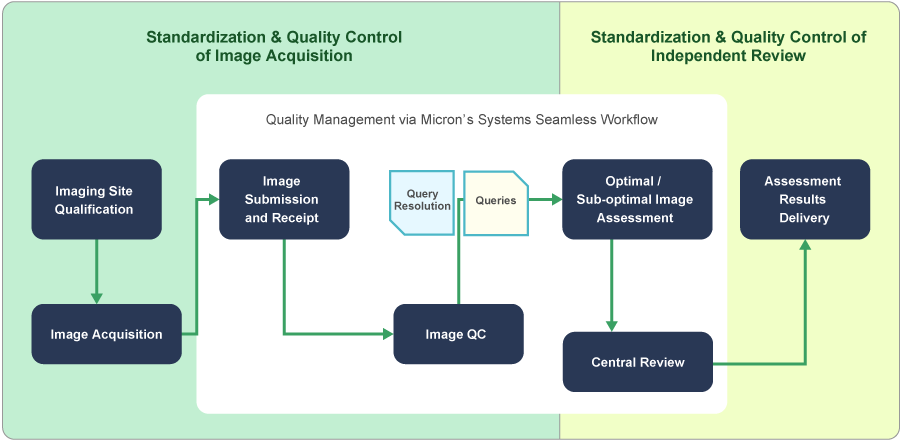
Metrics Monitoring Procedures
Micron monitors performance indicators in various imaging procedures with imaging performance metrics.
By confirming imaging metrics via Micron’s cross-trial quality management system, Micron evaluates imaging performance, uncovers any issues or errors, and accumulates the know-how to improve our methodologies.