The Ministry of Health, Labour and Welfare (MHLW) in Japan announced “DASH for SaMD” in 2020, a practical application promotion package strategy for medical device software (SaMD, Software as a Medical Device). While the environment for the practical application of SaMD has been improving mainly in Europe and the U.S., there had been concerns such as a “SaMD lag” (delay in practical application) in Japan. The objective of this package strategy is to promote the early commercialization of innovative cutting-edge SaMD.
|
DASH for SaMD
DX (Digital Transformation) Action Strategies in
Healthcare for SaMD (Software as a Medical Device)
|
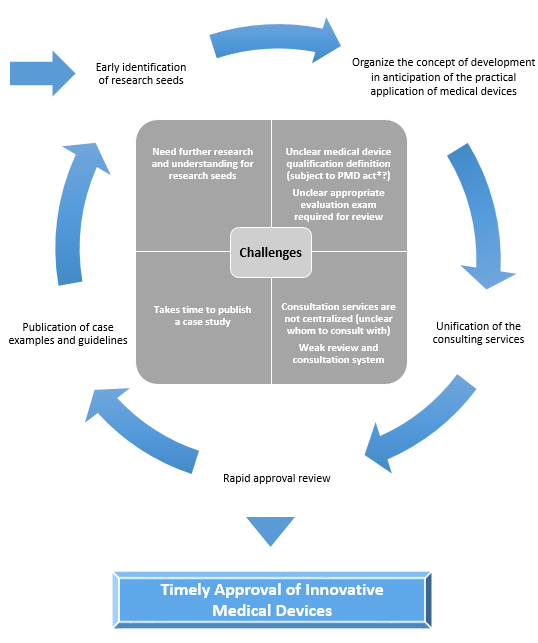
✓ Early identification of budding seeds of innovative SaMDs and presentation of the concept of review
✓ Establishment of a centralized consultation office and a review system and structure based on the characteristics of each SaMD.
Challenges and Policies for the Promotion of Practical Use
*PMD act: Japan’s Pharmaceuticals and Medical Devices Act
DASH for SaMD, SaMD Practical Application Promotion Package Strategy
|
1. Early identification of seeds and announcement of reviewing approaches
|
|
・Early identification of research seeds
・Assess the status of seeds in the early stage of development in Japan and abroad. Cooperate with PMDA.
・Organize and publish reviewing policy based on characteristics of SaMD
・Published review guidance’s. Cooperate with PMDA.
|
|
2. Unification of consultation service contacts
|
|
・Centralize consultation services
・Establish a centralized point of contact for consultations regarding the practical application of SaMDs and strengthen the coordination of the following various consultations.
・Consultation on determining whether software would qualified as a medical device
・Consultation on medical device development
・Consultation on medical insurance
・Publish case examples as possible
|
|
3. Review system based on characteristics of SaMD
|
|
・Carry out efficient review based on characteristics of SaMD
・Utilization of overseas data and advanced medical data, establishment of a system for pre-confirmation of quality management systems, etc.
・Utilize the Improvement Design within Approval for Timely Evaluation and Notice (IDATEN) scheme
・Prompt response to upgrades, etc. post approval
・Consider establishing the innovative SaMD designation program
・Reduce review period through priority consultation and review, enhanced pre-evaluation, and the review partner (concierge) system
|
|
4. Enhance structure for early realization
|
|
・Establish new section specialized in reviewing SaMD in PMDA and enhance structure of MHLW
・Establish an expert committee in the Pharmaceutical Affairs and Food Sanitation Council (PAFSC)
・Establish an industry-government-academia collaborative forum
・Enhance database of approval cases
|
Micron offers a full range of services for obtaining certification/approval of SaMD, including consultation, study planning and operation, and handling of PMDA approval applications.
Please feel free to contact us for more information.
Recommendations
Articles, Services and Solutions
Micron’s SaMD Support Package
SaMD Approval Support Achievements
References
DASH for SaMD (Publication from Ministry of Health, Labour and Welfare in Japan) Reference 1-3: Fundamental reform of the review of state-of-the-art medical devices such as medical device software. 24 November 2020, Ministry of Health, Labour and Welfare, Pharmaceutical Safety and Environmental Health Bureau (Japanese document)
Regulation system and perspective of SaMD OKAZAKI Yuzuru Director, Office of Software as a Medical Device, Pharmaceuticals and Medical Devices Agency (PMDA)
DASH for SaMD DX (Digital Transformation) Action Strategies in Healthcare for SaMD (Software as a Medical Device) Drastic reform of review system for innovative medical device including Software as a Medical Device, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare (MHLW), Japan

