Micron is actively supporting the development of medical device software that is increasing in demand, such as software for disease treatment. Micron has a strong track record in supporting medical device support development and can offer optimal and efficient processes.
Medical device software is also called SaMD (Software as a Medical Device). It is used to treat, diagnose, and prevent diseases. “SaMD” or “Software as a Medical Device” is defined by the International Medical Device Regulators Forum (IMDRF) as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
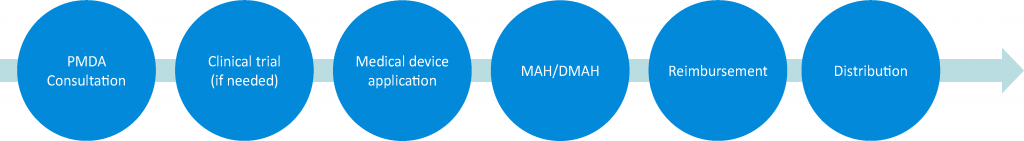
Micron has ISO13485:2016 certification and also a second-class medical device marketing license. Micron’s services and support packages include PMDA consultations, clinical trials, medical device applications, MAH or DMAH, reimbursement, and distribution. With a second class medical device marketing license, Micron can also be a MAH (Marketing Authorization Holder) or a DMAH (Designated Marketing Authorization Holder) for class 1 and 2 medical devices. We are the only CRO in Japan which can support you consistently from development support to sales with a second-class marketing license for medical devices.
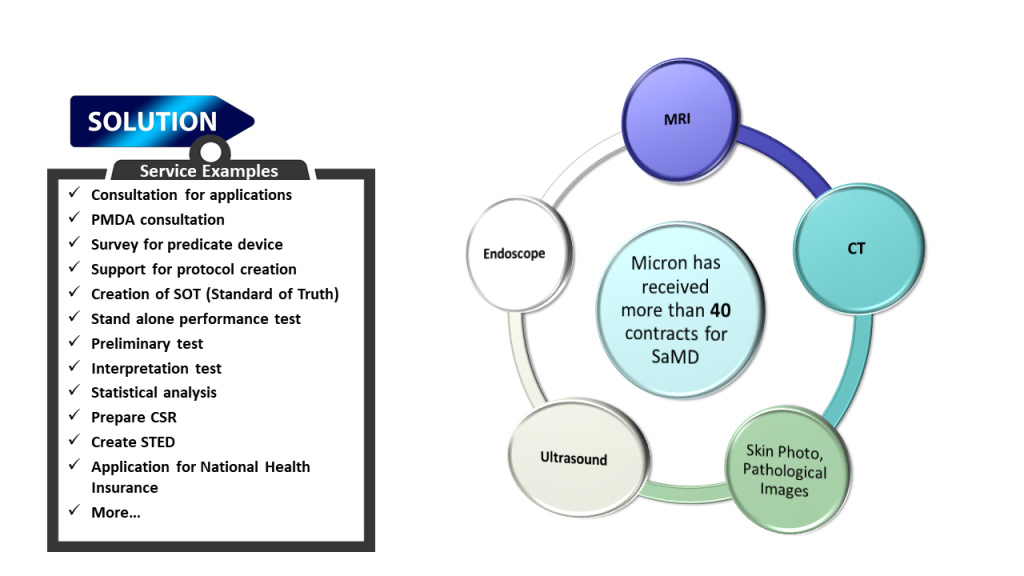
SaMD development often has unique requirements, in particular, disease diagnostic software often requires centralized image assessment (or centralized imaging interpretation) by radiologists and physicians. Micron has the expertise as an imaging CRO to conduct studies that meet reliability standards. The PMDA’s reliability inspection, which is conducted during the medical device approval review process, will be performed in details for SaMD as well.
Advances in AI technology have increased the performance of CAD (Computer-Aided Diagnosis/Detection), a medical imaging support system that uses AI, to a level that meets the expectations of clinical practice. As an imaging CRO, Micron has the know-how to create reference standards for medical images, assist in the design and implementation of performance validation tests, and analyze ROC. As a result, Micron is able to respond to a variety of requests.

Micron, which knows the development flow inside out, proposes the best and shortest process based on its experience and track record.
The company also offers various training services to meet requirements for each developing SaMD, including medical imaging diagnosis support software for which Micron is planning to apply for medical device certification or approval.
For SaMD development consultation, please contact a specialized CRO.
Reference
FDA, Food and Drug Administration, Software as a Medical Device (SaMD)
Recommendations
Articles, Services and Solutions
Regulatory Process for Medical Device Software in Japan
White Papers
The Development of Software as a Medical Device (SaMD): Clarification of Clinical Significance