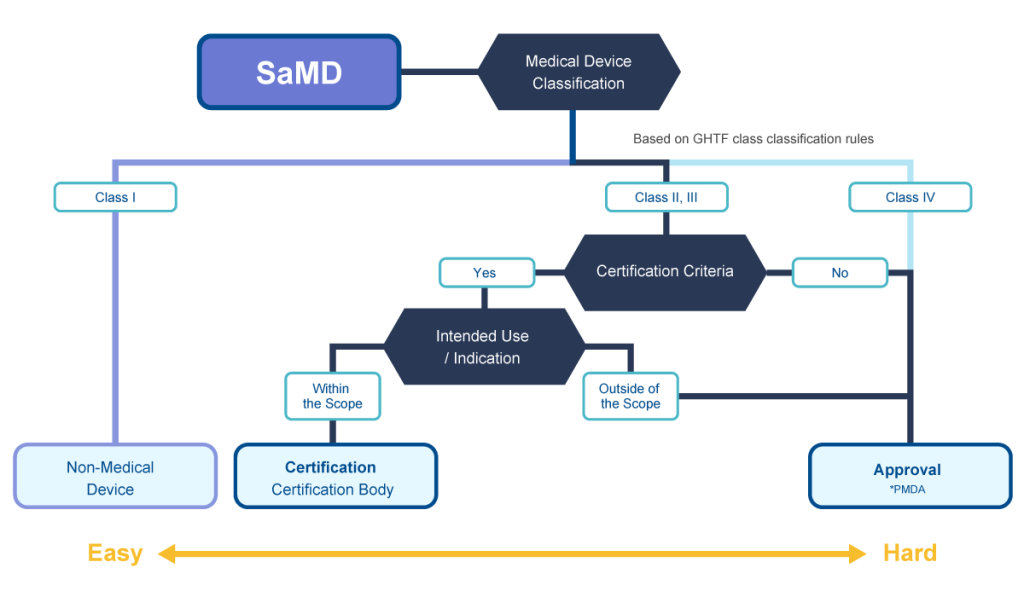
SaMD is software used for treatment, diagnosis and prevention of disease. This flow chart describes development and regulatory application processes in Japan.
*Pharmaceuticals and Medical Devices Agency
✓The need of clinical data greatly affects the time/budget required for development.
✓Micron can propose the optimal and shortest process.
Micron offers a full range of services for obtaining certification/approval of SaMD, including consultation, study planning and operation, and handling of PMDA approval applications.
Please feel free to contact us for more information.
Recommendations
White Papers
The Development of Software as a Medical Device: Clarification of Clinical Significance
Articles, Services and Solutions
SaMD Approval Support Achievements
The information contained in this document is subject to change without notice. Micron, Inc. makes no warranty of any kind with respect to this article (including, but not limited to, the implied warranties of merchantability and fitness for a particular purpose). Micron, Inc. shall not be liable for errors contained herein or for incidental or consequential damages in connection with the provision, performance, or use of this document.
This article was written on 27 Jun 2022.
